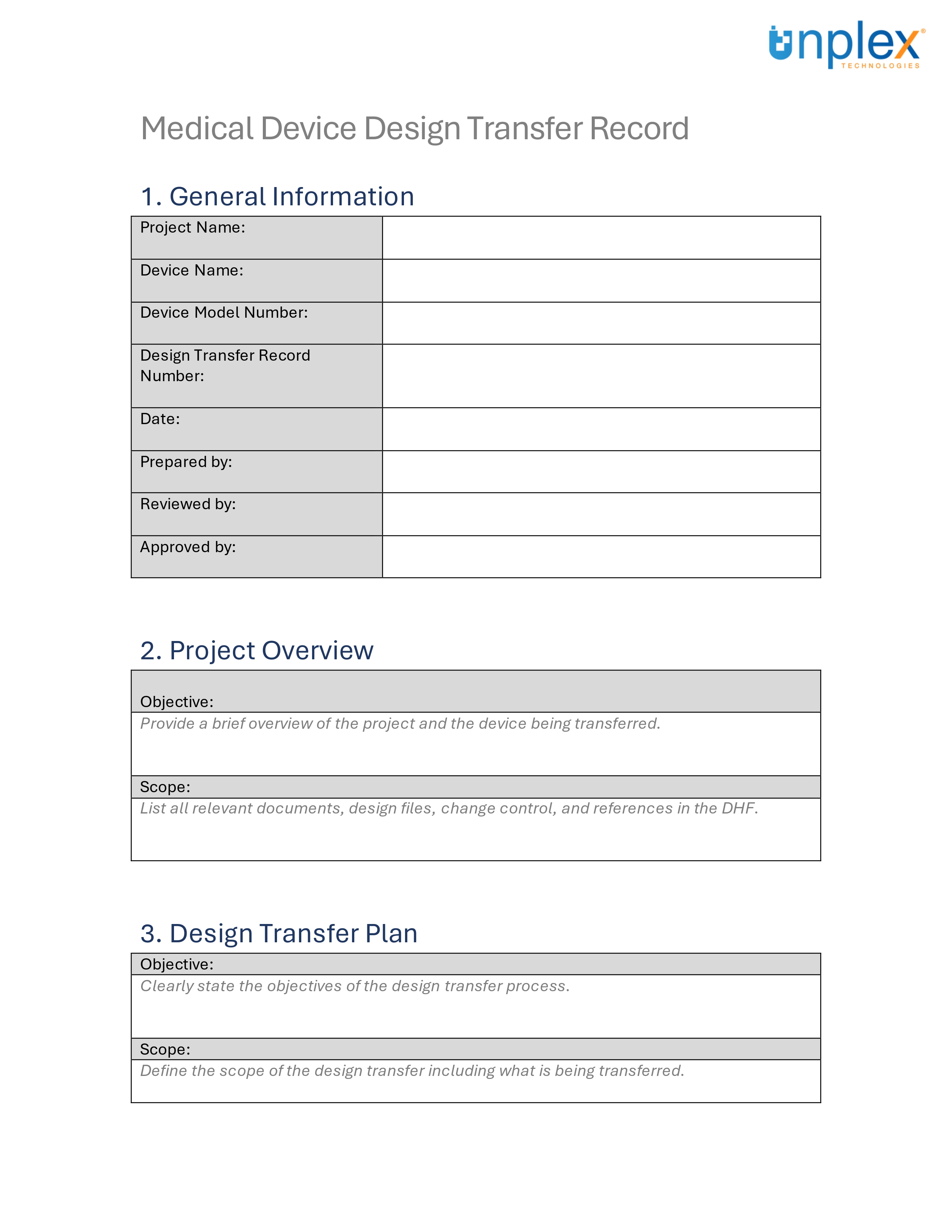
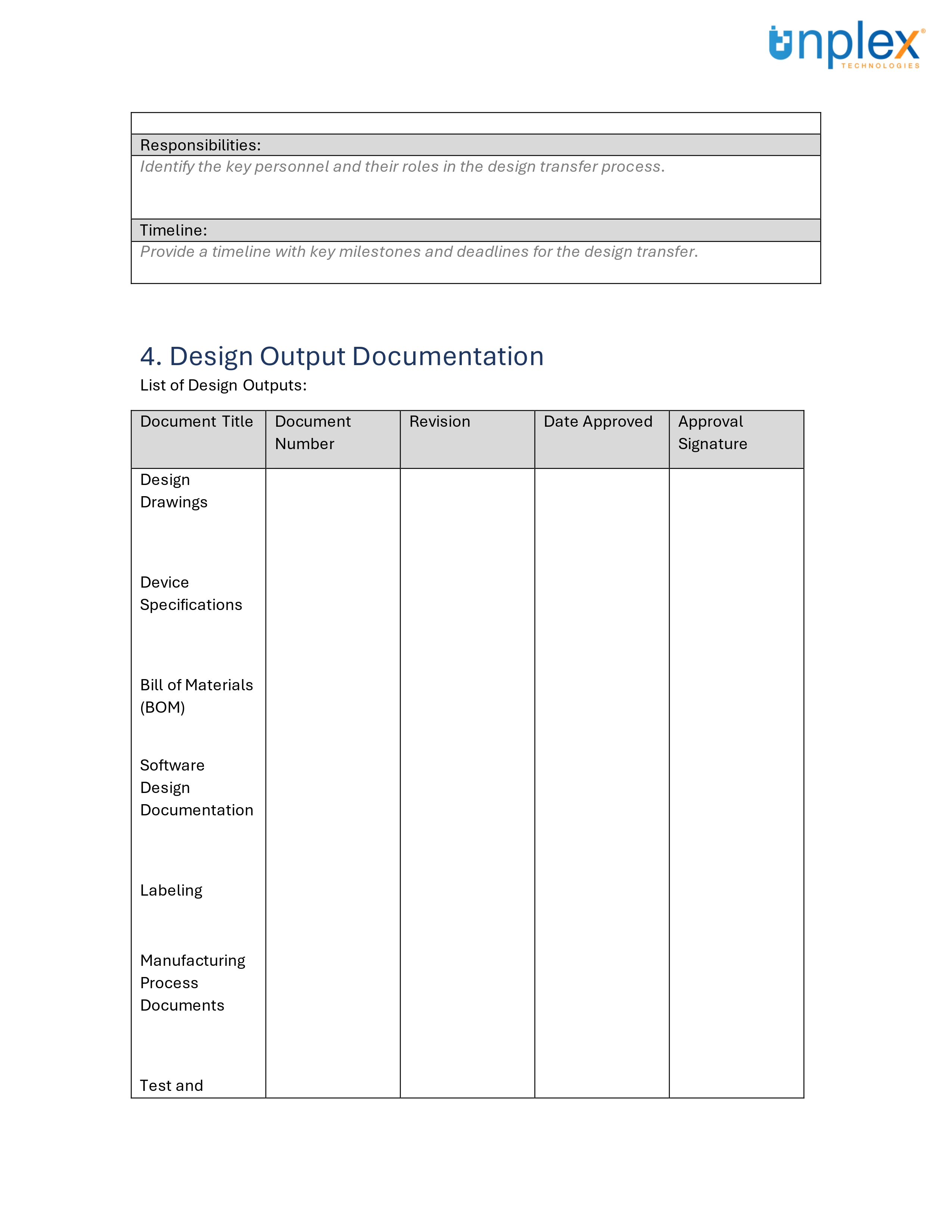
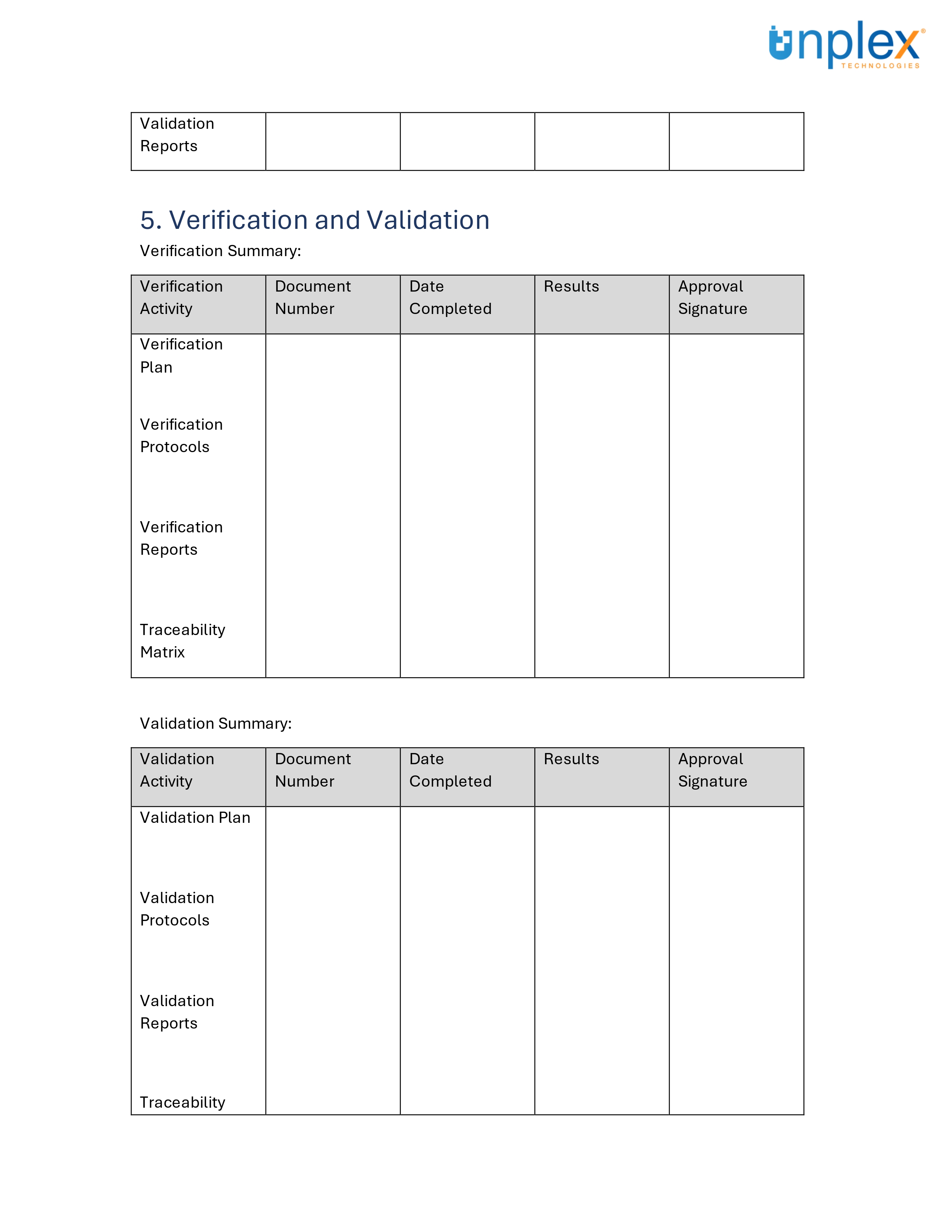
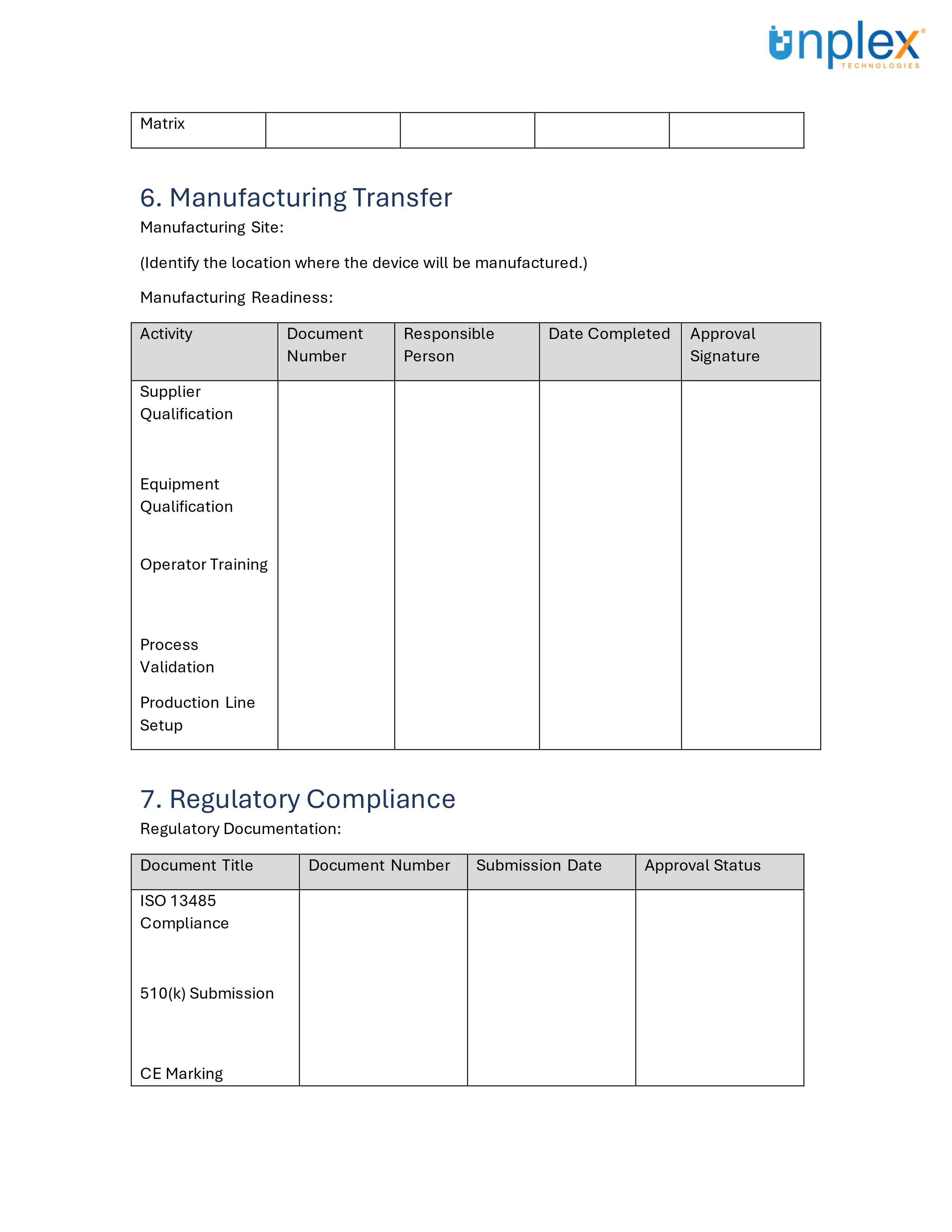
Design Transfer Record Template
Design transfer can be complex, but having the right tools can simplify the process.
To assist in ensuring a smooth and compliant design transfer, we've created a comprehensive template for medical device design transfer records.
This template covers all necessary documentation and signatures, aligned with ISO 13485:2016 and 21 CFR 820 requirements.
Streamline your design transfer process efficiently and effectively!