FMEA template
Here’s an FMEA template that can help you conduct thorough medical device risk assessments efficiently.
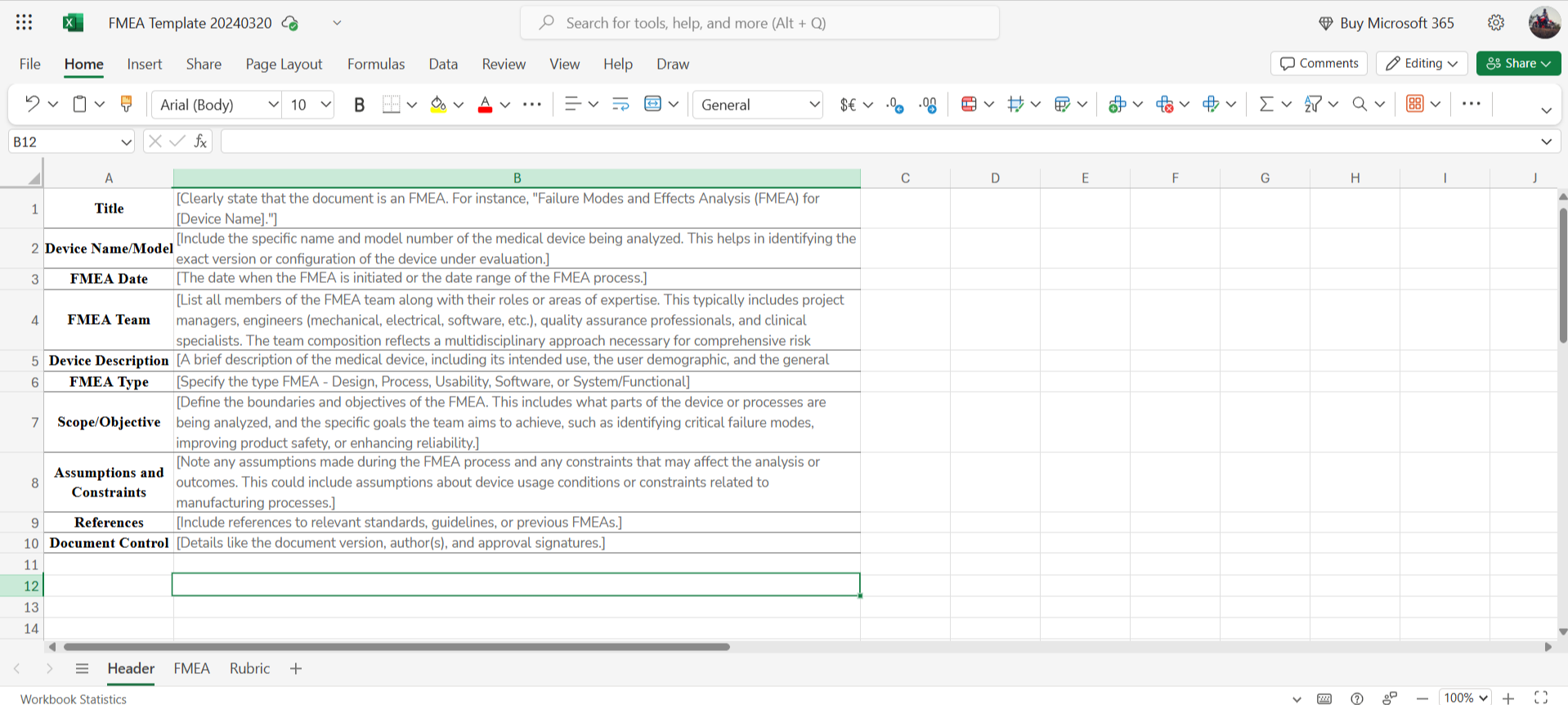
→ Header to include essential information such as device name, team details, and analysis scope, ensuring a clear understanding of the analysis context.
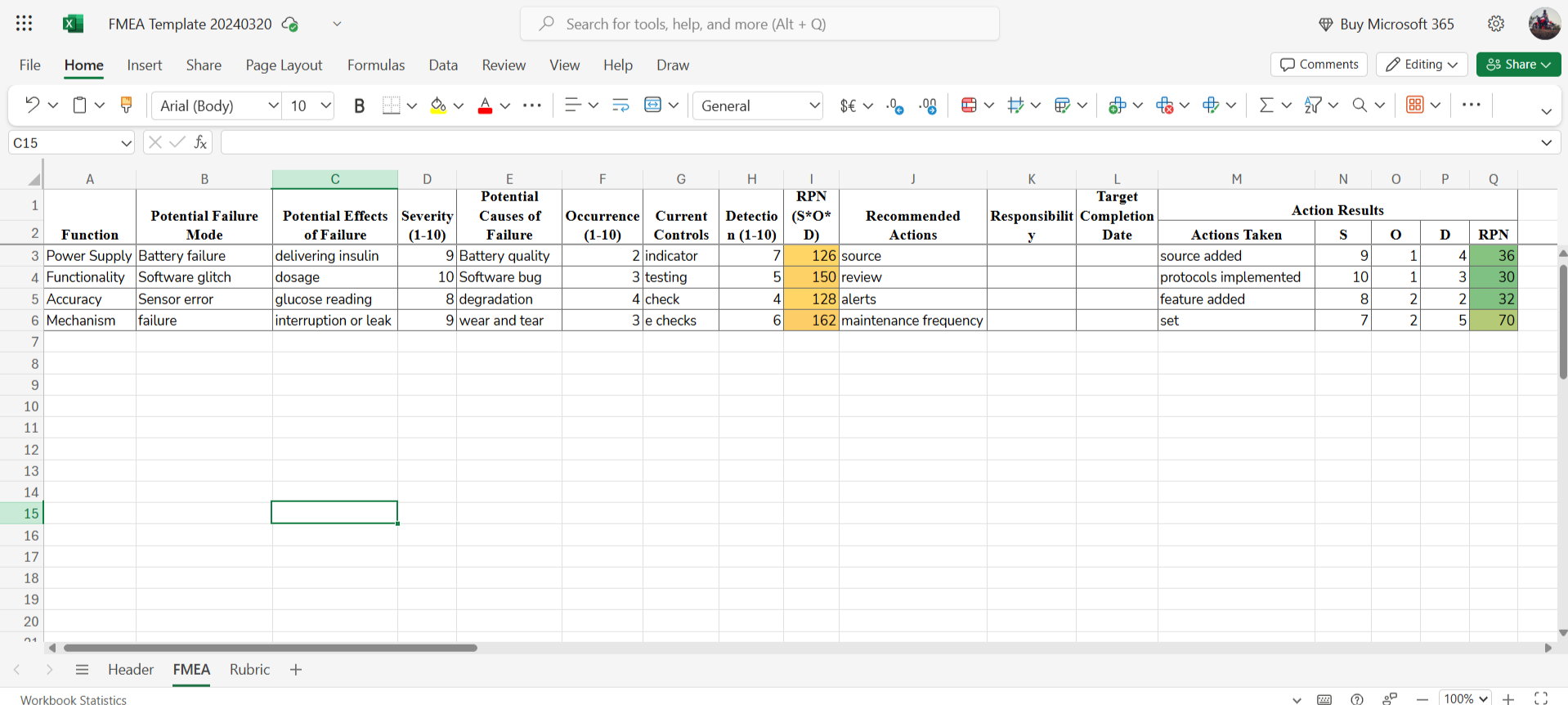
→ A detailed worksheet for evaluating potential failure modes, their effects, severity, occurrence, detection, and recommended actions, enabling a structured approach to risk management.
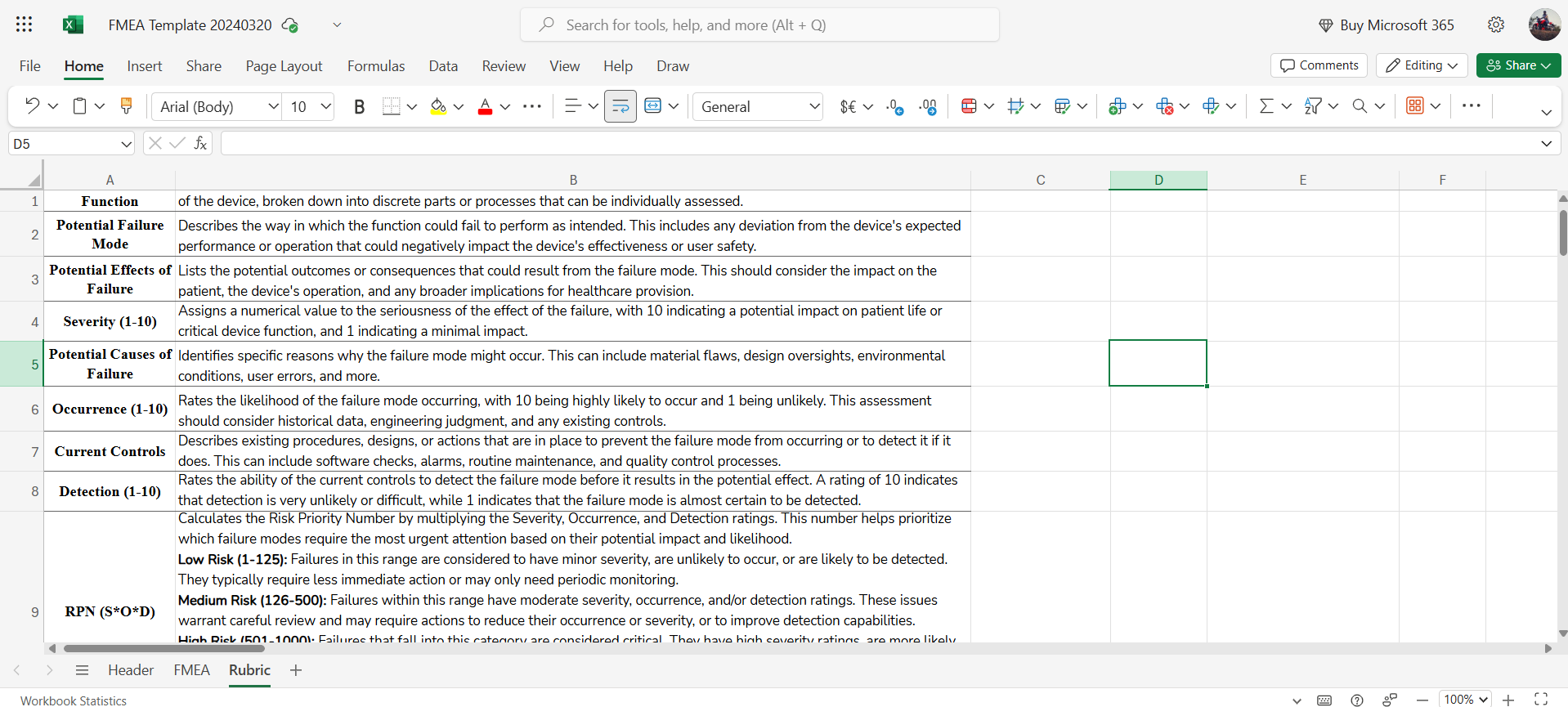
→ Rubric for clear definitions and scoring guidelines for severity, occurrence, and detection, ensuring consistency and objectivity in risk assessment.
Who should use it?
- Quality Engineers
- Design Engineers
- Process Engineers
- Project Managers
- Regulatory Affairs Professionals
- Anyone involved in the development, manufacturing, or quality assurance of medical devices
Feel free to use this template as a starting point for your FMEA activities and adapt it as necessary to fit your specific product needs and regulatory requirements.